
NPT Swivel Nut: How to Achieve Rotating Connections with a Tapered Thread
Imagine you’re installing a pressure gauge on a pipeline and, as you screw it in, you realize that when it finally tightens… the dial is
A controversial topic in our sector, it is truly one of those issues you don’t want to face when you receive a call about a nonconformity due to corrosion.
Next, I will briefly explain the catastrophic impact that galvanic corrosion can have with an example based on true events:
In 1763, the English frigate called “Alarm” suffered the consequences of galvanic corrosion. The situation was that they decided to apply a copper coating on the ship to protect the wooden hull from marine worms and barnacles. The problem arose when they attached the copper coating using iron nails. Due to the potential difference between these two elements and the presence of the electrolyte (saltwater), galvanic corrosion formed, endangering the ship’s functionality, structural integrity, and therefore the safety of the crew on board.
In this post, we will discuss galvanic corrosion, an electrochemical phenomenon that, if not considered, can have significant consequences on the durability and performance of metallic materials. It can ruin everything simply due to the poor selection of metals in contact with each other.

The following conditions must be met:
Therefore, as observed throughout this explanation, galvanic corrosion is highly relevant in industries that come into contact with electrolytes such as seawater. This includes industries such as shipbuilding, desalination plants, wastewater treatment plants, and any facility located near environments where saltwater is present.
But let’s get to the interesting part…
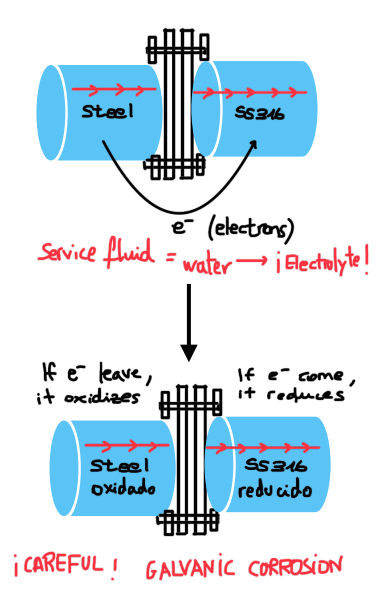
Next, we provide a system that allows you to accurately determine whether the combination of these materials will result in galvanic corrosion or if, on the contrary, you can forget about this problem.
At Redfluid, we offer a wide variety of materials for practically everything you’re looking for related to fittings and valves, whether for low or high pressure (a sector in which we specialize).
I hope everything is much clearer now, and you know, if you have any questions, feel free to contact us so we can clarify them for you 🙂
Share this post

Imagine you’re installing a pressure gauge on a pipeline and, as you screw it in, you realize that when it finally tightens… the dial is

Sounds familiar? The technician looks for a fitting, maintenance can’t find it, purchasing places an urgent order “for yesterday”… In the end, production gets delayed,